Background
This case study focuses on Applied Image’s work with a leading global CRO that provides various services from early development Phase II – III and pharmacovigilance to peri- and post-approval services. This CRO uses innovative scientific approach and analytical technologies to enable precise targeting and revolutionary clinical research.
As drug development and research equipment costs grow exponentially, this CRO uses its expertise and resources as a global partner to offer flexible solutions that help manage its customers’ costs and timelines.
In the early stages of pharmaceutical testing, microscopic work didn’t require a certified stage micrometer. Today, before researchers do microscopic work, the instrument must be verified with a certified stage micrometer. Stage micrometers have been around for a while. But now researchers are performing microscopic work with spectrometers, which necessitates unique stage micrometers for these instruments.
Casebook Situation
Drug product tablet testing for patient safety and compliance with manufacturing specifications has become increasingly complex. Most of these tests involve the dissolution of the tablet in a liquid solvent to evaluate critical aspects such as potency and purity. While these techniques can evaluate most aspects required by government regulators, each test has its own “blind spots” that necessitate evaluation by other methods.
Recently pharmaceutical scientists have realized the increased importance of evaluating drug product tablets in their original solid form. One technique researchers use to test drug tablets is Raman micro-spectroscopy. Raman spectroscopy involves a laser beam interacting with the molecules of a sample that researchers use for identification (qualitative analysis). Each molecule interacts with the laser uniquely to produce a spectrum with a unique “fingerprint” of peaks (see Figure 1 for spectral differences between aspirin and
acetaminophen).
About CRO
Contract Research Organizations or Clinical Research Organizations support the pharmaceutical, biotechnology, and medical device industries with outsourced research services. CROs are essential to help meet the needs of the evolving medical device and pharma industries. The leading CROs are focused on delivering innovative, life-changing therapies. They must uphold standards of operational excellence to support some of the world’s most groundbreaking development programs.
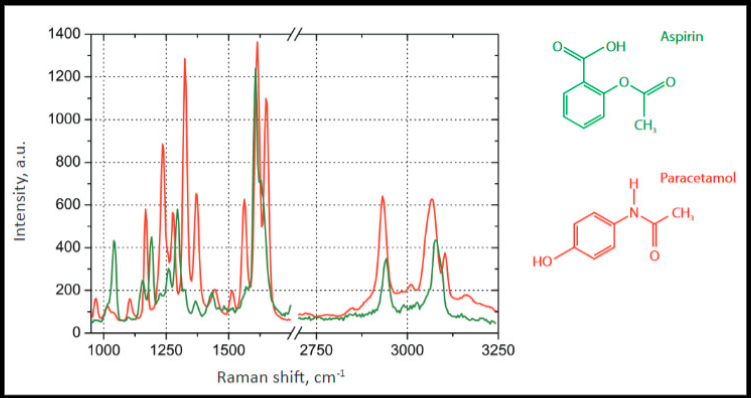
Figure 1: Overlay of Raman spectra of Aspirin and Acetaminophen (Paracetamol) showing differences in their spectral “fingerprint.”

Figure 2: Raman Microscope
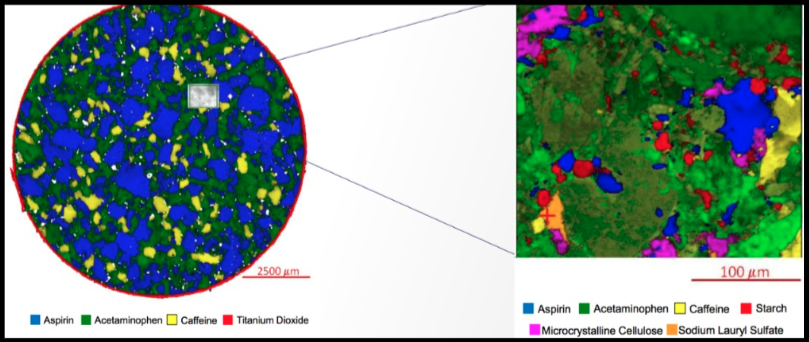
Figure 3: Raman Map of a tablet of Midol showing the distribution of active ingredients as identified by Raman spectroscopy.
Resolution
There isn’t a consensus in the pharmaceutical industry on certifying the size of features within a Raman map. It has been proposed that a microscope stage micrometer be manufactured with Raman active features that have had their size certified against a national standard. This proposal called out Applied Image as the only vendor in the world who can manufacture this type of micrometer. It is manufactured by taking a Raman active silicon wafer (commonly used in computer chips) and coating it with a Raman in-active chromium metal. Features are then created by laser etching the Chromium away to reveal the silicon wafer below it (see Figure 4 for the X/Y micrometer from our product).
With this unique micrometer, the pharmaceutical industry can utilize Raman maps to assess many additional aspects of drug tablets. For example, the total area percent of the aspirin in Figure 3 can be quantitated with traceability back to NIST as 52% of the tablet surface.
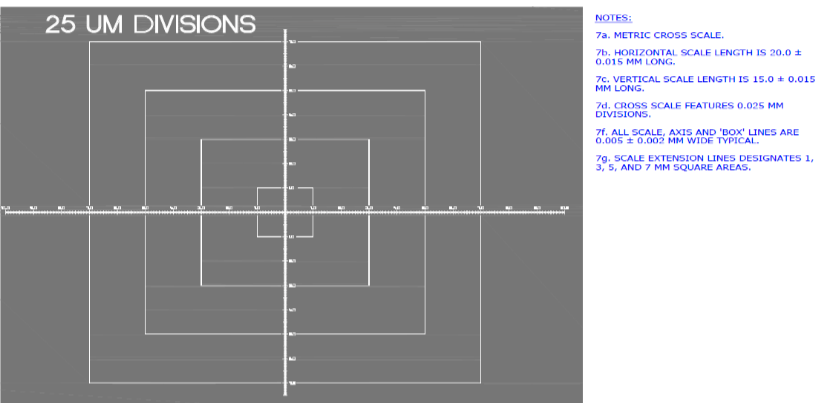
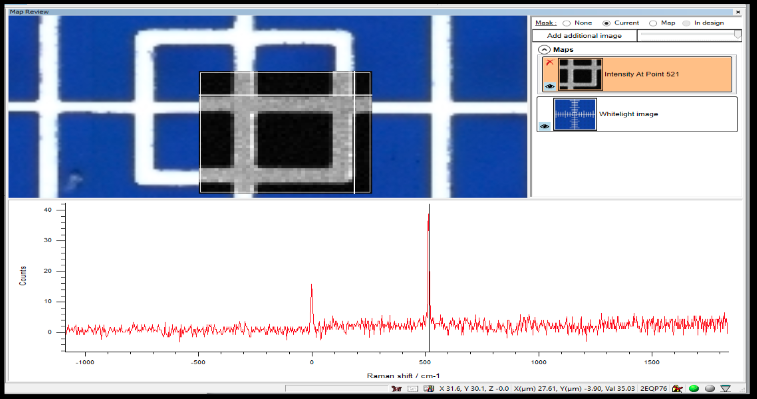
Why Was This Important to the CRO?
Having this one-of-a-kind Raman active stage micrometer with a high level of accuracy means the CRO can certify measurements within Raman maps. Contract research organizations invest in cutting-edge technologies to offer to their clients who aren’t ready to buy a $350,000 microscope that they may only use for a handful of projects over the year, only to sit idle the rest of the time.
CROs help pharmaceutical companies across the world avoid buying these instrumentsoutright. And so, in this case, research companies don’t have to make the development investment themselves when they have access to this custom stage micrometer produced by Applied Image. Pharma researchers can access unique, niche capabilities, as needed, without upfront investments.
Would You Recommend These Products/Services To Others?
The pharmaceutical research chemist leading the CROs project found Applied Image impressive in several ways. He first learned about Applied Image’s capabilities when he saw the company referenced in an ISTM document for Chrome on Silicon USFA 1951 resolution targets.
The chemist reached out to Darren Dixon, Sales Manager at Applied Image. The chemist learned that Applied Image specializes in developing some of the world’s most breakthrough precision-imaged designs, including components for NASA’s Mars Rovers. After speaking with Mr. Dixon, the CRO’s chemist realized that Applied Image was “one of the few companies in the world with the capabilities to develop the innovative component we envisioned.” Like this CRO, Applied Image offers high-quality, innovative, custom solutions to its customer. The team at Applied Image understood the applications that the CRO wanted to support. Their research chemist was delighted with the results, from the high-quality custom stage micrometer to the experienced team’s dedication to the project’s success.
Applied Image’s expertise in precision-imaged optical components with NIST traceable calibrations runs deep; it is the go-to company for producing revolutionary designs, whether you need one or hundreds of components.
Applied Image’s unique manufacturing processes and industry expertise allow us to create custom components and standards that meet the specific needs of our customers.
